How many more electrons does nitrogen need to satisfy the octet rule. What element has the electron configuration 1s22s22p3.
Nitrogen Electron Configuration Youtube
1s2 2s2 2p6 3s3 3p6 4s2 3d7.

. Element with electron configuration 1s2 2s2 2p3 is Nitrogen N What is 1s22s22p3. The electron configuration of nitrogenN and the orbital diagram is the main topic of this article. In addition to Nitrogen N an element in a configuration 1s2 2s2 2p3 has an electron.
1s2 2s2 2p6 3s3 3p6 4s2 3d7. The electron configuration of nitrogen is 1s2 2s2 2p3 How many more electrons does nitrogen need to satisfy the octet. The electrons that appear in the Lewis structure are the outermost electrons in the.
The electronic configuration of nitrogen is 1s2 2s2 2p3. Identify the inner electrons outer electrons and valence electrons in each electron configurations. It gives up electrons.
The electronic configuration of an element is the arrangement of its electrons in its atomic orbitals. Electron configurations should explain the structure of molecules. 1s2 2s2 2p3 potassium.
What element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2. What is N ground state electron configuration. 1s2 2s2 2p5 Neon configuration.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p1 3. The electron configuration for Copper Co is. 1s2 2s2 2p3 Oxygen configuration.
Thus nitrogen has a half-filled P orbital which is comparatively more stable. 1 2 Based on this what can you infer about the reactivity of helium and neon. 1s2 2s2 2p6 3s2 2p3.
What does 1s2 2s2 2p3 mean. Nitrogen has a 1s 2 2s 2 2p 3 electron configuration. 1s2 2s2 2p6 3s3 3p6 4s2 3d7.
The electron configuration shows that arrangement of electrons in an atomElectrons in atoms are arranged in orbitalsThe electron configuration of Nitrogen is 1s2 2s2 2p3. So an antimony atom with charge 2 has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1. Which one is correct electron configuration for a neutron atom of Nitrogen in excited state.
What does the S mean in 1s2. To achieve a stable gas configuration nitrogen needs to have a fulfilled p orbital. Experts are tested by Chegg as specialists in their subject area.
Therefore the answer is neon. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. Click card to see definition.
Nitrogen Element with electron configuration 1s2 2s2 2p3 is Nitrogen N It has two electrons in 1s two in 2s and three in 2p arbitrarily two in 2px and 1 in 2py 2. The electron configuration for Copper Co is. The electron configuration of nitrogen is 1s2 2s2 2p3.
Explanation of the many nitrogen oxides see in Wiki based on the unpaired electrons in degenerate molecular orbitals such as 1s2 2s2 2p3 for nitrogen configuration are. 1s2 2s2 2p6 3s2 3p4 8. What does 1s2 2s2 2p6 mean.
2p3 and the term symbol is 4S32. Answer 1 of 2. Those are the small number placed at the top after the letters.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10 4. Tap card to see definition. Given that the electron configuration for phosphorus is 1s22s22p63s23p3 answer the following.
The ground state electron configuration of ground state gaseous neutral nitrogen is He. What element is 1s22s22p63s23p6. The p orbital is partially filled with 3 unpaired electrons.
1s2 2s2 2p6 3s2 9. 1s2 2s2 2p3 6. Atoms of which element has the following electron configuration.
1s2 2s2 2p6 This represents 2 electrons in the s subshell of the first energy level 2 electrons in the s subshell of the second energy level and 6 electrons in the p subshell of the second energy level. We review their content and use your feedback to keep the quality high. The first two electrons will go in the 1s orbital.
How does oxygen obey the octet rule when reacting to form compounds. Who are the experts. To figure this out the element with the electron config of we first count the electrons.
Thus the p orbital is the outermost shell. The ground state electron configuration of carbon is 1s2 2s2 2p2. The closest noble gas neon Ne has the electronic configuration 1s2 2s2 2p6.
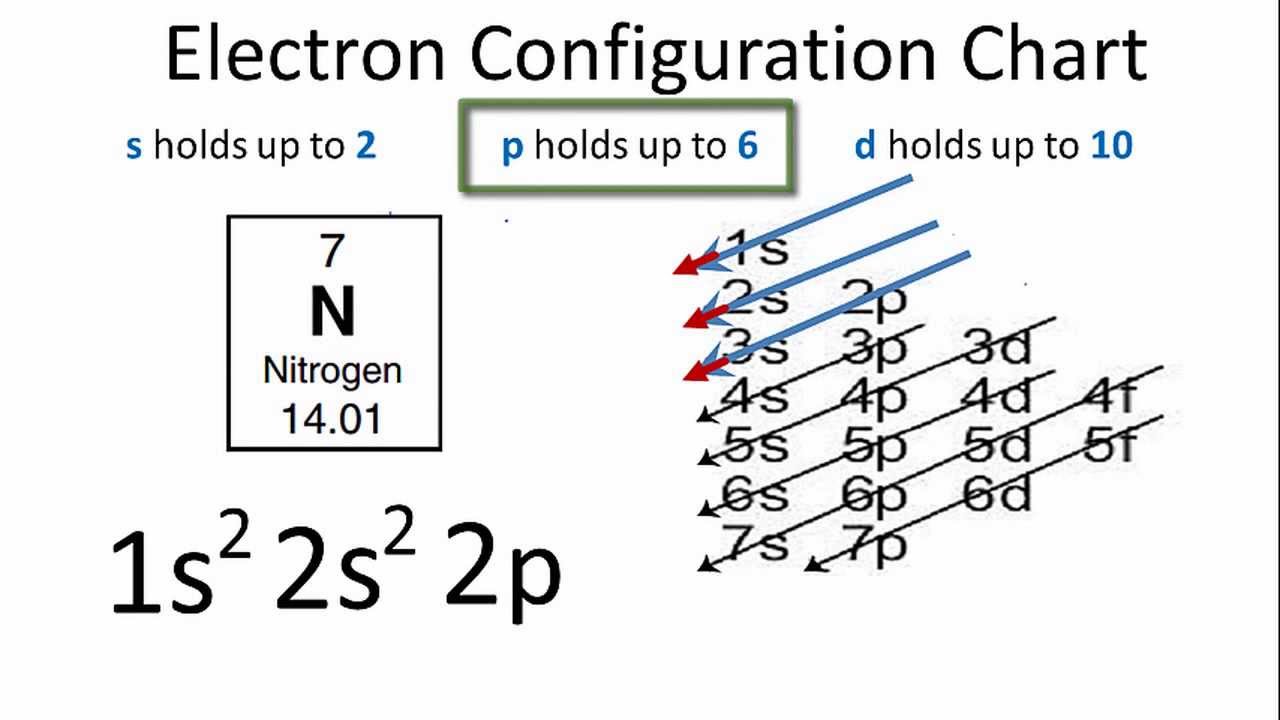
Nitrogen has an electron configuration of He 2s2 2p3. The atomic number of Nitrogen is 7 electronic configuration is 1s22s22p3. What element is 1s2 2s2 2p3.
1s2 2s2 2p6 3s2 3p6 5. The electron configuration of nitrogen atomic number 7 is 1s2 2s2 2p3More precisely it is 1s2 2s2 2px12py1 2pz1. 1s2 2s2 2p5 Your answer is given below.
How many of nitrogens electrons have ℓ 1. Element with electron configuration 1s2 2s2 2p3 is Nitrogen N It has two electrons in 1s two in 2s and three in 2p arbitrarily two in 2px and 1 in 2py. Nitrogen electron configuration is 1s2 2s2 2p3.
What electron configuration is 1s22s22p63s23p3. The electrons from the electron configuration that are part of the Lewis structure of N are 2s2 2p3. Element with electron configuration 1s2 2s2 2p3 is Nitrogen N It has two electrons in 1s two in 2s and three in 2p arbitrarily two in 2px and 1 in 2py 2.
The electron configuration for Copper Co is. Nitrogen atoms have 7 electrons and the shell structure is 25. Magnesium Mg is an element having electronic configuration 282.
1s2 2s2 2p4 Fluorine configuration.

Which Element Has The Electron Configuration Of 1s2 2s2 2p3 Youtube

Electron Configuration Ppt Download
What Is The Electronic Configuration Of Nitrogen In The First Excited State Quora
0 Comments